What is HEOR: Explained with Expert Insights
HEOR stands for Health Economics and Outcomes Research. It is a field of inquiry that brings together the disciplines of economics and patient-/population-oriented outcomes research. HEOR provides a framework that can clearly define healthcare issues and generate and assemble the relevant evidence to inform and guide healthcare-related decision-making.
HEOR looks at questions such as:
- What is the value of a healthcare intervention (drug, device, service) when you factor in its affordability, its clinical effectiveness, its impact on quality of life, and the broader system impact?
- Given limited healthcare resources (staff, funds, infrastructure), how do we decide what should be funded or prioritised?
- How do treatments perform in the real world (outside of tightly controlled clinical trials) in terms of outcomes, costs, and patient experience?
HEOR thus supports decision-making across payers, providers, industry and policy makers by providing multi-dimensional evidence: clinical, economic, and humanistic.

Nobel laureates Abhijit Banerjee & Esther Duflo, in an event conducted by the University of Sydney, said, "Good policy is grounded in evidence. It starts from understanding the world as it is, not as we wish it to be.”
In the event of their book launch, named “Good Economics for Hard Times”, they said, “When we told the government [in Indonesia] the results of our study, they basically printed those cards and mailed them to everybody. That touches the lives of 100 million people… When we succeed, the number of people affected is enormous. Time and again, we have seen in our travels in developing countries that hope is the fuel that makes people go. Defining people by their problems is turning circumstances into essence; it denies hope."
These insights illustrate that HEOR is not just about data and cost-effectiveness: it’s about translating evidence into policies that meaningfully improve lives on a large scale. HEOR informs resource allocation, shapes health policy, and centres on human well-being, ensuring interventions bring hope, dignity, and tangible benefits to patients and society.
Why HEOR matters in today’s healthcare landscape
Here are some of the key reasons why HEOR is highly relevant and increasingly central:
- Rising costs and finite resources: Healthcare budgets are under pressure globally, and decisions must be made about which interventions deliver the most health benefit for the cost. HEOR’s concept of scarcity and opportunity cost underpins this.
- Patient-centred and real-world focus: Traditional clinical trials focus largely on safety and efficacy under controlled settings. But HEOR emphasises real-world outcomes, patient-reported outcomes, and quality of life. This adds vital context about how interventions actually perform in routine practice.
- Market access and reimbursement dynamics: For pharmaceutical and medical device companies, generating HEOR evidence is key to securing reimbursement, pricing strategies, formulary listing, and access negotiations.
- Policy and system-level decision-making: Governments and Health Technology Assessment (HTA) bodies require robust evidence about cost-effectiveness, budget impact, and comparative value. HEOR feeds into those processes.
- Evolving treatments and complexity: With new therapies (personalised medicine, gene therapies, digital health), the stakes are higher. Understanding value beyond simple efficacy is essential. HEOR plays a pivotal role in that.
- Globalisation and emerging markets: Many low- and middle-income countries increasingly rely on HEOR to make coverage decisions, prioritise interventions, and use limited resources wisely.
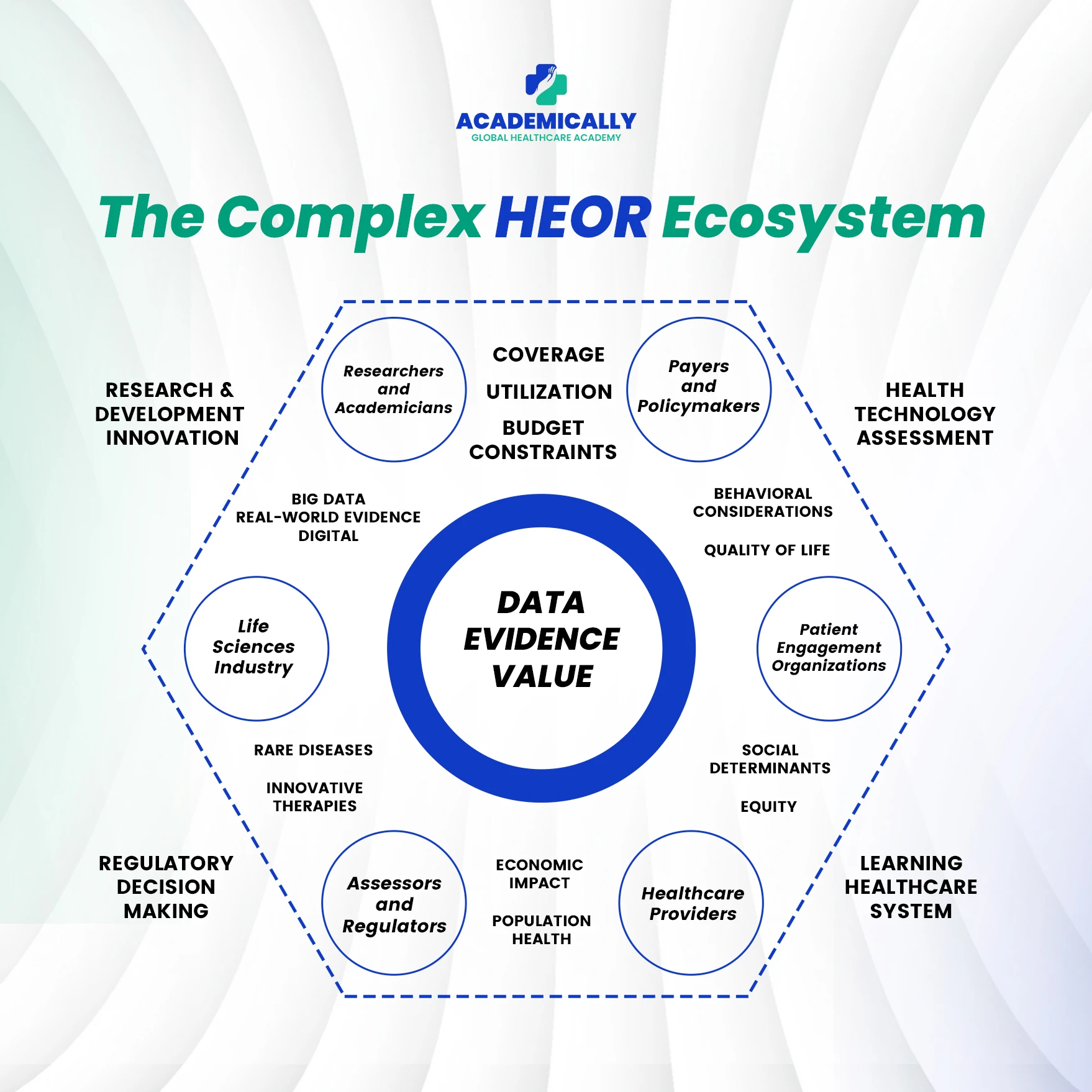
Key components of HEOR: Health Economics + Outcomes Research
HEOR is comprised of two major strands that overlap and synergise: health economics and outcomes research. Understanding both sheds light on how HEOR operates.
Health Economics
Health economics examines how scarce resources are allocated to maximise health outcomes. It involves analysing cost, benefit, efficiency, equity, and trade-offs. Health economics has to do with measuring and valuing the outcomes of healthcare interventions, including the behavioural patterns of healthcare market performance.
Key topics under health economics include:
- Cost-effectiveness analysis (CEA)
- Cost-utility analysis (CUA)
- Budget impact modelling
- Pricing, reimbursement and market access
- Resource allocation and value assessment
Outcomes Research
Outcomes research focuses on the results (clinical, economic, and humanistic) of healthcare interventions in real-world settings. It measures things like health status, quality of life, functional outcomes, resource use, and patient satisfaction. Outcomes research is the study of the results of specific healthcare practices and interventions. By analysing data from real-world settings, outcomes research helps to assess the effectiveness, safety, and value of these interventions.
Synergy
The synergy matters because you are not simply measuring outcomes or costs in isolation, but linking them. The outcome component ensures that you’re measuring what matters to patients and systems. The economics component places those outcomes into a decision-making framework of value, cost, and trade-offs. While both health economics and outcomes research can be performed in isolation, the synergy of combining the right data with thoughtful economic analyses ensures that even complex healthcare questions can be evaluated rationally.
The range of stakeholders and perspectives in HEOR
One of the reasons HEOR is complex and highly valuable is that multiple stakeholder perspectives must be considered. Each stakeholder has different objectives, constraints, and decision criteria. Some of the major perspectives include:
- Patients: What is the impact of the intervention on their quality of life, functional status, satisfaction, side effects and ease of use?
- Healthcare Providers (Hospitals, Clinics, Physicians, Nurses): How does the intervention influence clinical pathways, resource use, workflow, patient outcomes, adherence and readmissions?
- Payors (Insurance companies, government health programmes, private payers): What are the costs of the intervention (direct, indirect), what is the budget impact, what are the long-term savings (if any), what is the value delivered for population coverage?
- Industry (Pharma, MedTech, Biotech): How can the value proposition of a product be demonstrated (efficacy + economics + outcomes) to support pricing, access, launch strategy, and lifecycle management?
- Government / Health Technology Assessment (HTA) Bodies / Policy Makers: How do interventions align with national health priorities, budgets, equity goals, priority setting, reimbursement decisions, and cost-containment mandates?
- Society at large: What are the broader economic impacts (lost productivity, caregiver burden, societal cost of illness), equity considerations, and overall health system sustainability?
Methodologies and metrics used in HEOR
HEOR employs a range of methodologies and metrics to quantify and compare healthcare interventions. Below is a breakdown of key approaches and terms.
Cost-effectiveness analysis (CEA)
CEA compares the costs and outcomes of two or more interventions. The outcome is often expressed in terms of cost per unit of health benefit (e.g., cost per life-year gained). The famous metric is the incremental cost-effectiveness ratio (ICER)- cost difference divided by outcome difference.
Cost-utility analysis (CUA)
A method similar to CEA but uses outcomes weighted by utility (for example, quality-adjusted life years - QALYs). By putting quality and quantity of life together, CUA helps assess value in a more patient-centric way.
Budget impact analysis
This assesses how a new intervention affects the budget of a payer, health system or stakeholder over a defined time horizon, given forecast uptake and population dynamics.
Patient-reported outcomes (PROs) & quality of life (QoL)
Outcomes research emphasises PROs—how patients feel and function—and quality of life measures. Instruments can be generic (e.g., EQ-5D) or disease-specific. These feed into economic models.
Real-world evidence (RWE) & observational studies
HEOR uses real-world data: claims databases, electronic health records, longitudinal observational studies, and registries. These data reflect what happens in actual practice rather than in clinical trial ideal settings.
Modeling and decision analysis
Economic models simulate the long-term costs and outcomes of interventions, often when long-term real-world data are unavailable. Examples include Markov models, simulation models, sensitivity analyses, and probabilistic modelling.
Metrics and thresholds
Common metrics include: QALY, ICER, life-years gained, cost per QALY threshold, and net monetary benefit. Emerging metrics include alternative approaches such as evLYG (equal value of life years gained) and GRACE methods in some jurisdictions.
Sensitivity and uncertainty analysis
Given assumptions and data limitations, HEOR studies include sensitivity analyses (one-way, multi-way) and probabilistic sensitivity analysis (PSA) to assess robustness of findings.
Implementation and dissemination
Results of HEOR studies must be communicated to stakeholders: payors, HTA agencies, provider networks, and industry. Transparent methodology, strong data sources and tailored messaging enhance impact.
HEOR across the product lifecycle
One of the distinguishing features of HEOR is its role across the full lifecycle of a healthcare intervention. Here’s how it goes from early development through post-launch and beyond:
Pre-launch/development phase
- Identifying data gaps and planning for evidence generation (both clinical and real-world)
- Building economic modelling to forecast value, market access scenarios, and pricing strategy
- Defining patient-reported outcome measures and value proposition early on
Launch/market access phase
- Submitting dossiers to HTA agencies and payors with HEOR evidence (cost-effectiveness, budget impact, outcomes data
- Setting pricing and reimbursement negotiations backed by HEOR findings
- Designing real-world studies or registries to gather data post-launch
Post-launch/lifecycle management
- Real-world outcomes research: how the intervention performs in routine care
- Comparative effectiveness studies, long-term follow-up, and health economics updates
- Updating models based on actual data, refining value proposition, exploring new indications, expanding markets
Exit/renewal phase
- Continuing evidence generation to support renewal of reimbursement, generics, biosimilars, and new markets
- Reassessing value as the competitive landscape changes
- Using HEOR to support real-world contract models (value-based agreements)
The chance of securing access, optimising pricing and demonstrating ongoing value improves significantly by integrating HEOR early and across phases.
Real-world Evidence (RWE) in HEOR
Real-world data (RWD) claims databases, electronic health records (EHRs), registries and observational cohorts are increasingly central to outcomes research. HEOR uses these data to assess the performance of interventions in real-life settings. For example, studies show payors and formulary decision-makers already rely on HEOR evidence to make coverage decisions.
Benefits
- Captures diverse patient populations, comorbidities and real-world adherence
- Measures long-term outcomes, resource use, and cost implications outside trial settings
- Enables dynamic modelling, scenario analysis and evidence updates
Challenges
- Data quality, completeness and standardisation: missing data, non-compliance, and loss to follow-up pose methodological hurdles.
- Generalisability: Real-world studies may suffer from selection bias or confounders.
- Integration of multiple data sources and ensuring patient privacy and ethics.
- Evolving regulatory and payer requirements: agencies expect higher quality evidence, transparency and new metrics (for 2024 and beyond) in HEOR.
- Resource constraints in emerging markets: limited data infrastructure, less standardisation, and fewer HTA resources.
Best practices
- Plan HEOR evidence strategy early in product development: define outcomes, data collection, metrics and modelling in tandem.
- Use mixed-methods: merge clinical trial data, real-world data & modelling to build a robust case.
- Conduct transparent sensitivity and scenario analyses to reflect uncertainty.
- Report outcomes clearly for different stakeholder perspectives: payor, patient, societal.
- Keep updating evidence post-launch and communicate value consistently.
Global and regional considerations (emerging markets, India context)
While HEOR is well-established in many Western markets, its role in emerging economies and regions like India is growing and that too, with specific considerations.
- Different countries have different HTA frameworks, reimbursement thresholds, willingness-to-pay (WTP) benchmarks, and health system structures. Early research found large variability in HEOR use in Asia, Latin America and Africa.
- Metrics accepted in one jurisdiction may not be accepted in another (e.g., QALY may be restricted or replaced by alternative metrics).
- Data availability, infrastructure and regulatory frameworks vary significantly.
In India, the healthcare market is dynamic. Public and private sectors coexist, budgets are constrained, and demands for value-based care are increasing.
- Cost-of-illness assessments are vital for setting priorities in public health and private care.
- Real-world data generation in India may be less standardised; special efforts may be required to capture outcomes, quality of life, and patient-reported data.
- Developing HEOR capacity (skills, data analytics, modelling) is a growth area across the region.
- Local adaptation of models, thresholds and data sources is needed: imported models may not apply directly.
Future trends in HEOR
As healthcare continues to evolve, so does the field of HEOR. Key emerging trends that HEOR practitioners and educators should watch include:
Novel metrics & value frameworks
Traditional metrics such as QALYs may not be sufficient in all contexts. For example, alternative metrics like evLYG (equal value life years gained) and the GRACE methodology are gaining attention.
Greater use of AI, machine learning and real-world data analytics
Emerging reports highlight how generative AI and advanced analytics are being leveraged to enhance HEOR, but also emphasise the need for robust evaluation frameworks (such as the ELEVATE-AI framework) to ensure scientific rigour.
Value-based contracts and outcomes-based reimbursement
As payors shift towards outcomes-based payment models, HEOR will need to deliver evidence that supports these models: linking payment to real patient outcomes.
Patient-centric outcomes and equity
There is increasing focus on not only average outcomes but distributional outcomes: equity, access, health disparities, and variation by socio-economic status. HEOR will incorporate more societal and caregiver cost data.
Globalisation and local adaptation
HEOR will need to adapt to different regional contexts, data environments and local health systems. Emerging markets present unique challenges and opportunities.
Digital health, wearables, real-time outcomes
As digital health grows, real-time data from wearables, remote monitoring and patient apps will feed into outcomes research and economic models. HEOR professionals will increasingly need to integrate these new data streams. By staying ahead of these trends, learners and practitioners of HEOR can be better prepared for the future landscape of healthcare decision-making.
As HEOR continues to shape healthcare policy, pricing, and patient outcomes across the world, the demand for skilled professionals who understand the science and application of health economics and outcomes research is rising sharply. Yet, despite its growing importance, HEOR remains an area where structured, practical education is often limited, especially in regions like India and other emerging markets.
Our vision is to bridge the skill gap between academic learning and professional practice. The HEOR programme embodies this mission of empowering healthcare professionals, pharmacists, data analysts, and life sciences graduates to become part of the evidence-driven future of healthcare.
To Conclude with…
HEOR is no longer an optional adjunct in healthcare or life sciences decision-making. It is foundational. For anyone involved in product development, policy, reimbursement, or healthcare education, mastering HEOR opens doors to more insightful, evidence-based decision-making. At Academically, we are committed to equipping you with the knowledge, tools, and real-world perspective you need to thrive in this domain.
If you’re ready to deepen your understanding of HEOR and boost your capability in health economics and outcomes research, speak with our expert team for personalised insights.





