Did you know that 68% of managers within pharmaceutical and biotech firms are planning to expand their MSL teams within the next two years? With this upward momentum, the role of the Medical Science Liaison (MSL) is more critical than ever and also increasingly competitive. Are you a life-science professional? Or are you an employer drafting a job requisition? In this blog, we are talking about a medical science liaison job description. It will entail day-to-day responsibilities and required skills, career progress, industry outlook and more. Keep reading.
Who is a Medical Science Liaison (MSL)?
MSL is a scientifically trained professional who serves as the link between the pharmaceutical/biotech/medical-device company and an external medical community of clinicians, researchers, key opinion leaders or KOLs.
An MSL establishes and maintains peer-to-peer relationships with leading physicians at major academic institutions and clinics. Therapeutic specialists with advanced scientific training are experts in communicating complex scientific and medical information to a variety of stakeholders. The role emphasises “education rather than sales” and focuses on being the scientific expert for specific drugs/devices.
Why the role of MSL is growing?
Here are a few reasons the MSL role is growing:
- Complex product launches in specialty and precision medicine require deep scientific engagement and external expert collaboration.
- The regulatory and scientific environment demands credible external engagement and evidence generation beyond just commercial messaging.
- As companies increasingly focus on patient access, real-world evidence (RWE), and healthcare economics, the MSL becomes a key player bridging medical affairs, clinical development and external stakeholders.
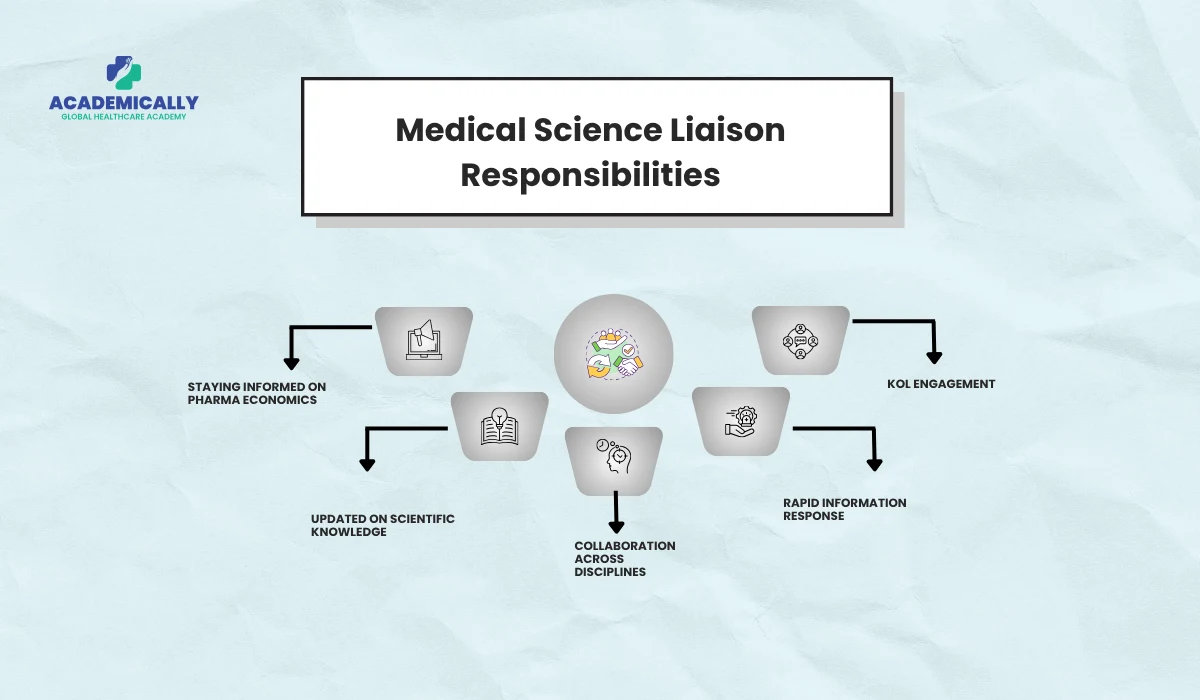
Job Description: What Does an MSL Do?
When companies write job adverts, they usually ask: “What does a Medical Science Liaison do?” Here’s a comprehensive breakdown of the core activities. So if you are an MSL looking for a job, bookmark this to have an idea of what’s in store for you.
External Engagement & Scientific Exchange
Identify, map and build relationships with
- Key Opinion Leaders (KOLs)
- Thought leaders
- Academic physicians
- Researchers
- High-value clinical sites.
Conduct peer-to-peer scientific discussions about
- Therapeutic areas
- Mechanisms of action
- Clinical trial data
- Pipeline products
- Real-world evidence, and
- Best-practice patient management.
Respond to unsolicited medical enquiries from
- Healthcare professionals
- Distributors or
- Internal colleagues, providing fair, balanced, up-to-date medical information.
- Represent the company at scientific congresses, symposia and advisory boards: deliver presentations, host discussions and gather feedback.
Internal Collaboration & Strategy
Serve as the scientific expert internally:
Translating external insights (from KOLs, clinicians, etc.) into actionable inputs for cross-functional teams (medical affairs, clinical development, regulatory, commercial).
Support the development of the
- Medical plan
- Evidence generation strategy
- Advisory boards
- Investigator-initiated studies (IIS)
- Real-world evidence projects and launch readiness.
For example, the role description at Novartis mentions identifying data gaps and contributing to integrated evidence-generation plans. - Train or support internal teams (marketing/sales/commercial) on the scientific and medical aspects of the therapeutic area, ensuring compliance and accuracy.

Insight Generation & Feedback Loop
- Collect and synthesise field-insights: clinical practice trends, unmet medical needs, competitor products, patient journeys and physician perspectives.
These insights help shape strategy, brand planning, product development and market access. - Monitor scientific literature, medical guidelines, and emerging research, and communicate implications to both external and internal stakeholders.
Therapeutic Expertise & Scientific Credibility
- Maintain deep knowledge of the assigned therapeutic area (e.g., oncology, immunology, cardiovascular, neurology, rare diseases).
This includes the mechanism of action, drug pipeline, clinical guidelines, real-world evidence, health economics and biomarker landscape. - Interpret clinical trial design and data, epidemiology, and statistics that translate complex data into understandable messages tailored to different audiences (clinicians, internal teams, and regulatory).
Compliance and Ethical Conduct
- Operate within the regulatory context: non-promotional scientific exchange, adherence to guidelines (e.g., PhRMA code, local regulations), ensure accuracy, balance and transparency of information.
- Ensure all activities are compliant, especially when interacting with external stakeholders, responding to enquiries, participating in advisory boards or trials.
Field-based and Travel-heavy Responsibilities
- Many MSL roles include frequent travel, a geographic territory to cover (hospitals, academic centres, clinics), often outside the office. For example, one Indian job advert noted 70-80% travel.
- Balance between field time and desk-based responsibilities (preparation, training, internal meetings, strategy) is key.
Breakdown by Lifecycle Phase & Focus Areas
One of the things that distinguishes the best job descriptions is acknowledging that the MSL role varies depending on the lifecycle of the product, the therapeutic area, and the type of company. Let’s break that down.
Early Development Phase
In early drug development (pre-launch), MSLs might engage external experts to build awareness of the science, mechanism of action, unmet need, biomarker strategy, and potential future applications. They may engage in investigator-initiated trials, liaise with clinical sites, support protocol design and engage KOLs in pipeline discussions.
Purpose: build a scientific foundation so that when launch comes, external stakeholders are aware and engaged.
Launch/Post-Launch Phase
The MSL supports the launch of a product: explaining clinical trial results, real-world evidence, facilitating advisory boards, supporting investigator-led studies, and providing medical education.
They also monitor uptake, access issues, payer/pricing landscapes, and gather insights from sites on how the product is performing. They become strategic partners for commercial teams to ensure appropriate product adoption, but always remaining in the medical-affairs (non-promotional) sphere.
Late Lifecycle / Real-World Evidence / Market Access Phase
MSLs may shift focus to real-world evidence, patient journey mapping, health economics outcomes research (HEOR), working with access teams on barriers to patient care, expanding indications, and building long-term relationships.
They may lead post-marketing observational studies, work on lifecycle management, label expansions, and capture evolving scientific data to maintain the scientific credibility of the company.
By Therapeutic Area
- Oncology, immunology and rare diseases: heavy scientific complexity, high unmet need, frequent external expert interaction.
- Cardiovascular or diabetes: may involve broader physician networks, more structured education efforts.
- Medical devices: may require deep technical understanding of device mechanics, user training, and hospital procurement pathways.
Skills, Qualifications & Experience: What Companies Look For
When writing or evaluating the job description for a Medical Science Liaison, it’s important to list the required and preferred qualifications and skills. Here’s a comprehensive view.
Educational Background
- Most MSL roles now require an advanced degree: PhD, Pharm.D, MD, or equivalent in the life sciences. The MSL Society survey indicated 77% of U.S. MSLs hold a doctorate.
- In some regions/companies, a Master’s degree plus strong field/clinical experience may be accepted, but competitive roles often list a doctorate as preferred
- A strong therapeutic area focus in the academic credentials (e.g., oncology research, immunology, neuroscience) is often highly desirable.
Work Experience
- Prior experience in clinical research, medical affairs, the life science industry, or as a clinician can be an advantage.
- Experience presenting at scientific meetings, publication record, involvement in investigator-initiated studies or real-world evidence generation is often highlighted.
- Field experience: ability to travel, engage with senior clinicians, navigate hospitals/academia and multi-stakeholder environments.
Core Skills and Competencies
Technical Skills:
- Ability to interpret and translate clinical trial data, understand study design, statistics, pharmacology, and disease biology.
- Strong therapeutic expertise in the assigned area.
- Knowledge of regulatory, ethical and compliance frameworks regarding scientific exchange.
- Presentation, training design, scientific and communication skills.
Soft/Interpersonal Skills:
- Excellent relationship-building skills, especially peer-to-peer with KOLs and internal stakeholders.
- Strong communication (oral and written) and networking capability.
- Critical thinking, curiosity, the ability to synthesise information, and problem-solving.
- Self-motivation, agility, ability to work independently in the field and remain business-oriented but scientifically credible.
Salary Expectations & Location Variability
- UK MSLs earn around £50,000; experienced MSLs £60,000–£80,000; up to £100,000+ for senior roles.
- US roles tend to have higher salary bands, factoring in bonus/stock; one source listed ~$160k national average in the US.
- In India, you can earn around 24 LPA to 48 LPA.
A Day in the Life of an MSL
| Time | Activity |
| Morning | Travel to a regional academic hospital; meet with a KOL to discuss the latest clinical data and patient needs. |
| Midday | Lunch with another clinician or a hospital department, sharing a scientific update and hearing feedback on treatment patterns. |
| Early Afternoon | Back at home office or remote: review field insights from earlier meetings, prepare internal report, update CRM. |
| Late Afternoon | Internal meeting with medical affairs and commercial teams: discuss insights, align on advisory-board planning, and discuss upcoming congress attendance. |
| Evening | Prepare presentation slides for a scientific dinner meeting tomorrow; review the latest literature in the therapeutic area; maybe respond to HCP enquiries received. |
Tips for Building a Strong Resume to Crack a Job Interview for MSL
- Highlight therapeutic area expertise and any publications, conference presentations, or research.
- Showcase your ability to engage in peer-to-peer conversations; not just “sales”, but scientific exchange.
- Provide examples of insight generation, collaboration with cross-functional teams, and how you translated data into action.
- Emphasise travel, field experience, territory management, and the ability to work independently.
- Demonstrate communication/presentation skills, both written and verbal.
- If you’re transitioning from academia/clinical research, speak to how your work is relevant to the industry context (clinical trial data, evidence generation, patient-outcomes improvements).
Future Outlook: How to Proceed
The medical science liaison role offers various advancement paths:
- Entry-Level MSL → MSL → Senior MSL → Lead MSL/Regional MSL → Medical Affairs Manager/Director → Head of Field Medical/Medical Affairs.
- Some MSLs transition into broader roles in brand strategy, development, access or even global medical roles.
- In the UK, senior MSLs can earn over £100,000, sometimes evolving into associate medical director roles.
First step to become an MSL
Given the strong demand for high-quality MSL professionals and the increasing complexity of therapeutic landscapes, upskilling is more than a differentiation; it’s a necessity.
Why you should consider further education:
- Deepens your therapeutic expertise and industry understanding beyond academic research.
- Builds credibility in the industry, showing you understand the MSL role, field medical responsibilities and bridging skills between science and business.
- Enhances readiness for field-based roles (travel, cross-functional collaboration, stakeholder engagement) rather than purely lab or academic roles.
- Equips you with hands-on insights into medical affairs, evidence generation, KOL engagement, real-world data and value communication.
Good luck on your journey to becoming an outstanding MSL!
To Conclude With…
The medical science liaison job description is rich and multifaceted: scientific expert, educator, strategist, field ambassador and insight generator. Because the role sits at the nexus of science, clinical practice, business strategy and stakeholder engagement, it demands strong credentials, well-honed skills and field readiness. The role varies depending on therapeutic area, product lifecycle, company type and region. However, the core remains consistent: build trusted scientific relationships and translate evidence into impact. For professionals looking to enter the MSL field, strategic upskilling (such as our PG Certificate in Medical Science Liaison) makes a big difference. For hiring managers, crafting a clear, comprehensive and specialised job description helps attract high-quality talent who understand the role and can deliver. With the growing demand for MSLs globally, now is a great time to position yourself or your organisation for success.





