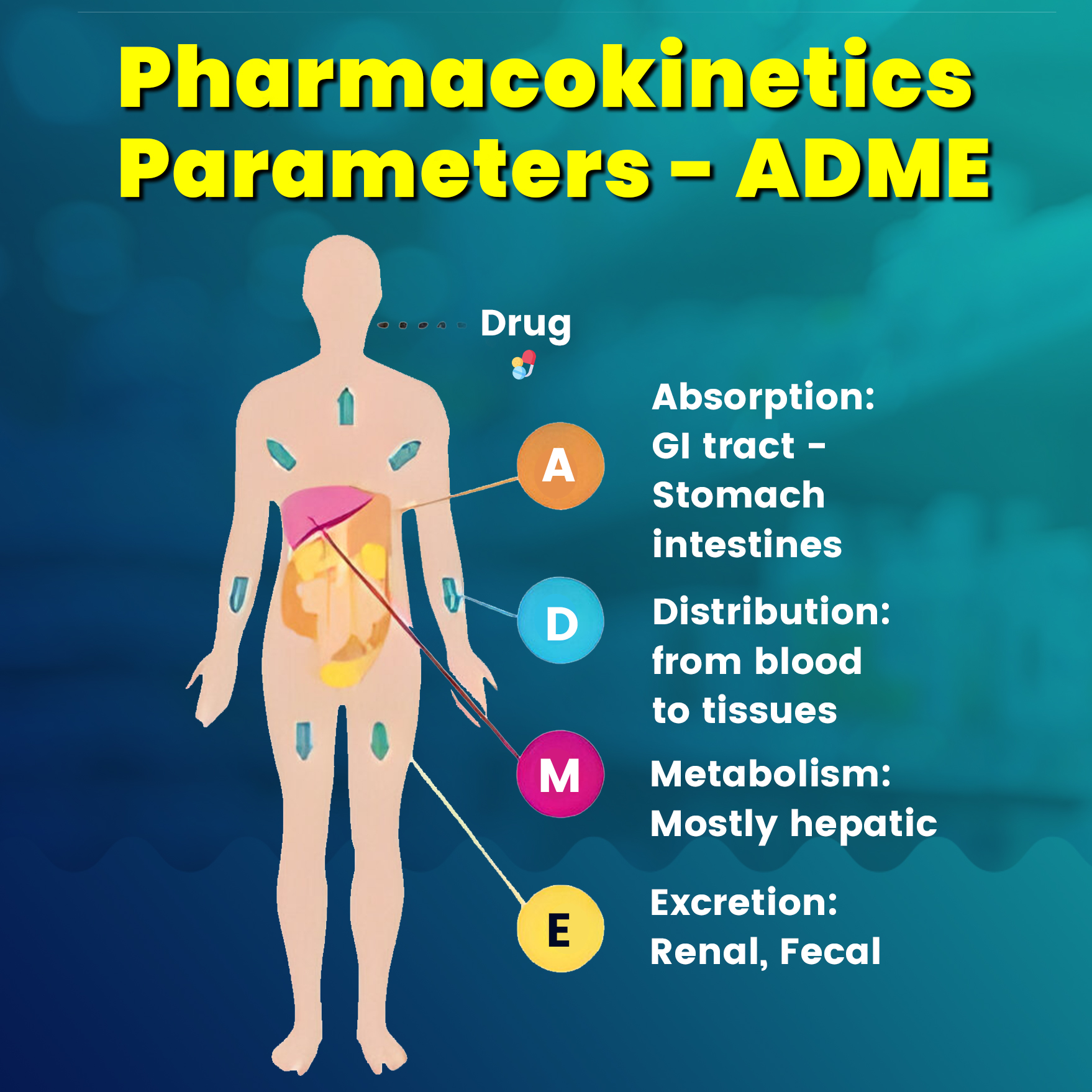
Absorption
The movement of drugs from the site of administration into the bloodstream is known as absorption.
Factors influencing drug absorption
1. Physicochemical properties of the drug:
- Physical state: The liquid form of the drug is better absorbed than solid formulations.
- The drug's lipid-soluble and unionised form is better absorbed than the water-soluble and ionised form.
- Particle size: Drugs with smaller particle sizes are absorbed better than larger ones.
- Disintegration time: The time taken for the formulation (tablet or capsule) to break up into small particles, and its variation may affect the bioavailability.
- This dissolution time is the time taken for the particles to go into the solution. The shorter the time, the better the absorption.
2. Route of drug administration: A drug administered by intravenous route bypasses The process of absorption as it directly enters the circulation. Some drugs are highly polar compounds, ionise in solution and are not absorbed through the GI tract. Hence, they are given parently.
3. PH and ionisation: Strongly acidic and strongly basic drugs usually remain ionised at all pHs, so they are poorly absorbed.
4. Food: the presence of food in the stomach can affect the absorption of some drugs.
5. Presence of other drugs: concurrent administration of two or more drugs may affect their absorption.
Bioavailability
- The fraction of administered drug reaches the systemic circulation in the unchanged form.
- When we administer a drug, it is absorbed into the portal circulation and reaches the liver. Here, some drugs may be metabolised (first-pass or systemic metabolism), and the rest reaches systemic circulation. Absorption and first metabolism are two important determinants of bioavailability.
- By the IV route, it is 100%.
Bioequivalence
If the difference in the bioavailability of the two preparations (same drug, same dose, same dosage forms) is less than 20%, these are not to be bioequivalent.
Distribution
Distribution is defined as the reversible transfer of drugs between body fluid compartments. After absorption, a drug enters the systemic circulation and is distributed in the body fluids.
The apparent volume of distribution is defined as the hypothetical volume of the body fluid into which the drug is uniformly distributed at a concentration equal to that in plasma, assuming the body to be single.
Lipid-soluble drugs are more likely to cross the blood vessel wall and thus have a high volume of distribution. If a drug is highly bound to plasma proteins, it will behave like a large molecule and likely stay in the plasma. Therefore, less will go to the tissues, resulting in a reduced volume of distribution.
Blood-brain barrier
Placental barrier
Certain drugs administered to pregnant women can cross the placenta and affect the foetus or newborn. Quaternary ammonium compounds, e.g., d-tubocurarine, and substances with high molecular weight, like insulin, cannot cross the placental barrier.
Metabolism
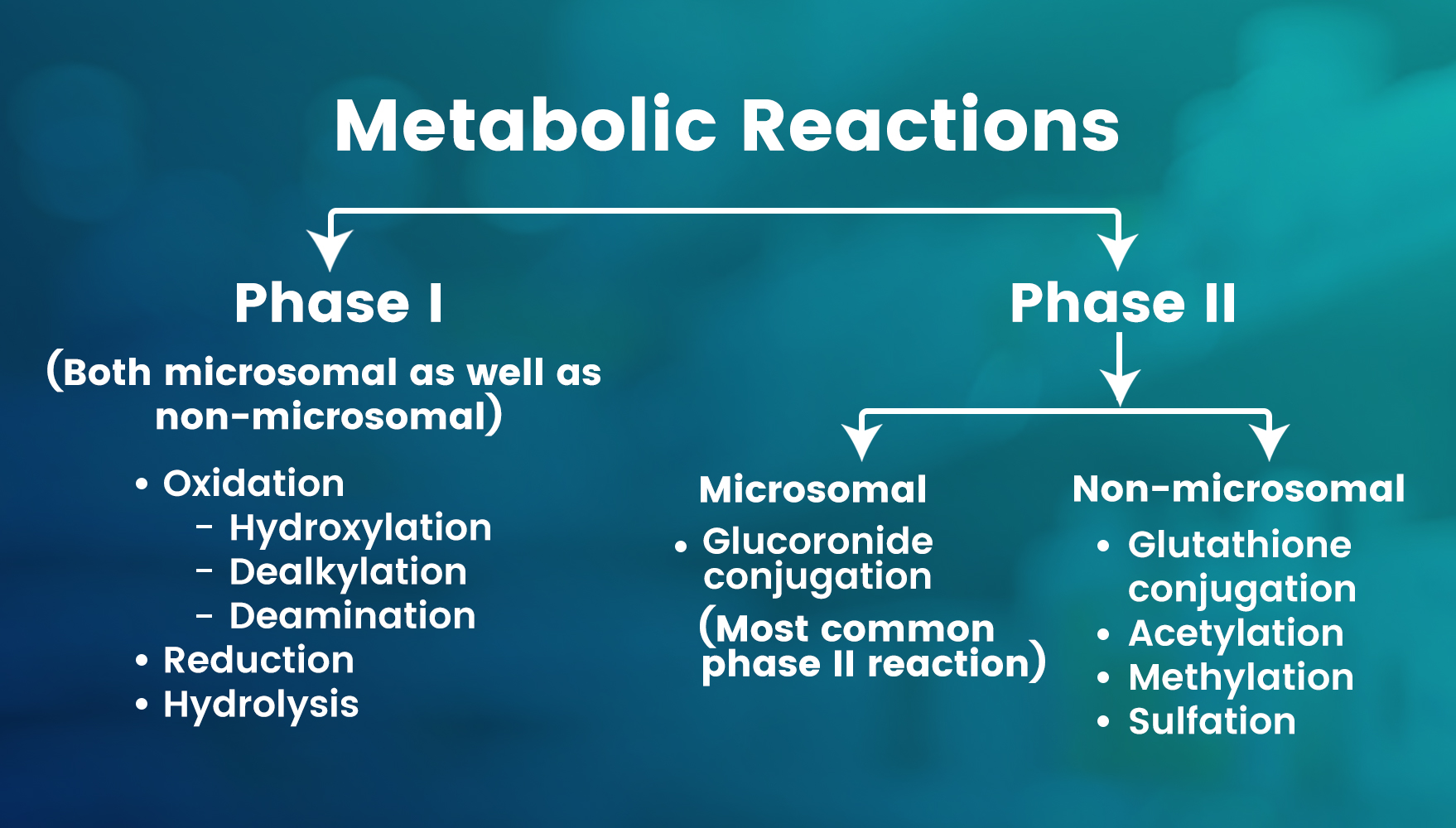
Metabolism may occur with the help of microsomal (present in the smooth endoplasmic reticulum) or non-microsomal enzymes. Other drugs may induce or inhibit microsomal enzymes (monooxygenases, cytochrome P450 and glucuronyl transferase), whereas non-microsomal enzymes are not subjected to these interactions.
Metabolic reactions may be classified into phase I non-synthetic and phase II synthetic reactions. The function of the phase I reaction is to attach a functional group to the drug molecule, whereas the phase II reaction serves to attach a conjugate to the drug molecule.
After the phase I reaction, the drug may be water-soluble or soluble, whereas after the phase II reaction, all drugs become water-soluble (lipid insoluble).
Excretion
The major route of excretion is the kidney. Excretion through the kidney occurs by glomerular filtration, tubular reabsorption, and tubular secretion.
Glomerular filtration depends on the binding of plasma protein and renal blood flow. It does not depend on lipid solubility because all substances, whether water-soluble or lipid-soluble, can cross their fenestrated glomerular membrane.
Tubular reabsorption depends on lipid solubility. If a drug is lipid soluble, more will be reabsorbed and less excreted. The opposite is true for insoluble drugs.
Tubular secretion does not depend on lipid solubility or plasma protein binding. In the nephron, separate pumps are present for acidic and basic drugs. Drugs utilising the same transporter may show drug interactions.
Final Words
Pharmacokinetics is the study of how the body affects a drug, which is crucial for optimising drug therapy and ensuring patient safety. By understanding the processes of absorption, distribution, metabolism, and excretion, pharmacists and healthcare professionals can make informed decisions on drug dosing and administration.
Academically has courses specially designed for various pharmaceutical exams like the KAPS Exam Preparation Course, PSI Examination Course, and SPLE Exam Preparation Course.
Please fill out this form to talk to our experts and choose the country and exam of your choice.