Did you know that roughly 40% of all clinical trials worldwide are now managed by Contract Research Organisations? Yes, that’s right. The global pharmaceutical and biotech sectors accelerate growth by several driving factors. These range from rising demand for faster drug development, real-world evidence and digitally enhanced clinical research. This is the reason the need for full-service CROs is a lot more than usual. This growth is translating into massive hiring across the industry. Whether you're a fresh graduate, data-oriented professional, regulatory affairs aspirant, or just exploring entry-level roles in clinical operations, there’s never been a better time to look at CROs.
In this expert-curated guide, we will talk about the top CROs actively hiring globally and in India. You’ll also get to know what roles you can expect, and how you can position yourself to grab those opportunities.
Why CROs Are Hiring
- Market expansion & rise in outsourcing:
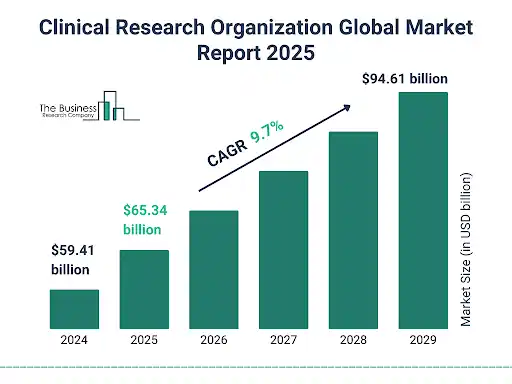
The global CRO services market is expanding steadily. According to a recent report, the market, valued at USD 100.01 million in 2025, is expected to grow to USD 111.33 million in 2026, on its way toward USD 292.26 million by 2035. - Rising trial volume, complexity & therapeutic breadth:
As drug development expands to new therapeutic areas (rare diseases, oncology, biologics) and as regulators globally call for more rigorous data, CROs are increasingly in demand for their ability to handle complex, multi-phase trials, safety monitoring, and regulatory compliance. - Geographical diversification India & APAC are trending hubs:
While North America and Europe still hold the majority share of CRO activity, Asia-Pacific, especially India, is seeing a surge, thanks to cost-efficiency, trained workforce, and a growing number of global trials being outsourced. - Technology & real-world evidence (RWE), AI and data analytics integration:
As data-driven trials, decentralised trials, and hybrid models take centre stage, CROs with strong analytics, real-world-data capabilities, and digital-first infrastructure get a competitive edge and, accordingly, are hiring for data, biometrics, and technology roles.
For job-seekers and early-career professionals, this rising demand across specialties means diverse entry paths, not just traditional CRA (Clinical Research Associate) or study-coordinator roles, but data-oriented, analytics, regulatory, and remote/hybrid-trial roles.
Top Global CROs Hiring Aggressively in 2026
Here are some of the biggest names globally, well-established CROs with diverse therapeutic and service offerings, who continue to ramp up hiring.
IQVIA
- IQVIA remains one of the largest CROs worldwide, with a workforce of about 88,000 across 100+ countries.
- Their business integrates clinical trial management, R&D solutions, analytics, and real-world data (RWE), making them a powerhouse for roles in data analytics, biostatistics, real-world evidence, health-data science, and global clinical operations.
- For candidates: IQVIA is known for structured onboarding and early-career programmes (site management, regulatory affairs, PV data entry, coding analyst roles) in markets including India.
Parexel
- An established CRO specialising in late-phase, full-service clinical research, regulatory consulting, medical writing, data management, and pharmacovigilance.
- Parexel has a strong global presence, with dozens of facilities worldwide.
- For freshers and early-career aspirants, Parexel is known to run dedicated fresher/training programmes (statistical programmers, associate programmers), especially in biometrics and data-heavy roles.
Syneos Health
- A full-service CRO that integrates both clinical and commercial capabilities; helpful for firms seeking end-to-end support from drug development to market launch.
- Syneos regularly hires for biometrics roles (SAS programmers, data analysts), clinical operations, project management, and more.
ICON plc
- Based in Dublin, with a global presence as of 2024, ICON had ~41,900 employees across 55 countries.
- Offers full-service CRO solutions covering all phases of clinical research; known also for adaptive trial designs and leveraging digital health platforms for faster, quality-driven trials.
- For India and other growth geographies, ICON frequently opens junior programmer, graduate trainee, and entry-level roles, a good fit for freshers and early career professionals.
Labcorp Drug Development / Fortrea (formerly Covance)
- Labcorp (and its spun-off CRO arm Fortrea) ranks among the major full-service CROs globally, handling preclinical to post-approval trials, and offering capabilities in data analytics, regulatory services, and safety monitoring.
- Fortrea (formerly Covance) is also notable for infectious-disease and vaccine trials, animal safety/toxicology studies, and global clinical trial management.
- They often offer entry-level roles in statistical programming, data management, and clinical data operations, making it a good platform for candidates looking to start their career with a full-service CRO.
Charles River Laboratories
- With over 20,100 employees globally as of 2024, Charles River is a giant in preclinical and early-phase CRO services, offering safety assessment, toxicology, research models, and early clinical support.
- For drug development pipelines that require robust preclinical-to-early-phase work (e.g., animal-model research, safety/toxicology, regulatory compliance), Charles River remains a global favourite.
Medpace
- A full-service CRO with a strong specialised focus on oncology and rare diseases. It partners frequently with small-to-mid-size biotech companies and offers clinical development services across all phases.
- Its growth trajectory has been solid, with increasing revenues and expanding presence globally, offering opportunities for data management, project operations, and clinical trial coordination.
Top Indian CROs Hiring in 2026 That You Should Know
While global CROs dominate the headlines, India remains a rapidly growing centre for outsourced clinical research, owing to cost-efficiency, regulatory maturity, a growing patient pool, and a skilled workforce.
Here are CROs within India that are especially active and relevant for job-seekers.
- Syngene International: As a subsidiary of a major biopharma company, Syngene offers integrated R&D services including clinical trial units, bioanalytical labs, and support for multinational studies. They are well-known for Phase I trial capabilities and regulatory-compliant clinical infrastructure.
- Veeda Clinical Research: Focuses heavily on bioequivalence (BE), bioavailability (BA), early-phase studies, and has approvals/inspections from major global regulators (US FDA, EMA, etc.). They are increasingly expanding into Phase II/III studies and eClinical platforms, which translates into hiring in clinical operations, data management and regulatory documentation.
- Lambda Therapeutic Research: A global-footprint CRO headquartered in Gujarat, with strong services in pharmacovigilance, data management, regulatory support, and clinical operations, making them an attractive employer for early-career and mid-level professionals.
- Cliantha Research: Known for focusing on therapeutic areas like dermatology, ophthalmology, and metabolic diseases; offers integrated services (clinical, bioanalytical, safety) and is recognised for quality trial execution, signalling ongoing hiring in niche therapeutic trials.
- Accutest Research Laboratories: Specialises in BE/BA studies, early-phase clinical trials, and analytical labs. Their reputation for regulatory compliance and efficient turnaround makes them a relevant option for lab analysts, CRAs, QA, and data roles.
- Others / Established Indian CROs: Several other India-based CROs have been active historically (e.g., SIRO Clinpharm, Synchron Research Services, smaller niche CROs) and periodically open roles in data, pharmacovigilance, lab services, and clinical operations.
What Types of Roles Are in Demand in CROs
Based on the hiring trends across these CROs, here’s a breakdown of the most in-demand roles valuable for aspirants coming from varied backgrounds (life sciences, pharmacy, data science, regulatory affairs).
| Function / Role Type | Why It’s in Demand | Typical Employers / CROs |
| Data Science & Biometrics (SAS programmers, statistical programmers, data analysts, RWE/data analytics) | Increasing integration of real-world data, AI, and analytics; decentralised/hybrid trials demand data-heavy workflows. | IQVIA, Parexel, Syneos Health, ICON, Labcorp/Fortrea, Medpace |
| Clinical Operations & Project Management (CRAs, Study Coordinators, Project Managers, Site Management) | More trials, broader therapeutic areas and multi-country studies need operational manpower. | Global CROs + Indian CROs (Syngene, Veeda, Lambda, Cliantha, Accutest) |
| Regulatory Affairs / Pharmacovigilance / Compliance | Stringent global regulation, post-marketing surveillance, safety monitoring, especially in multi-national trials. | Parexel, Labcorp, Indian CROs expanding BA/BE or Phase II–IV trials |
| Bioanalytical & Lab Services / Preclinical / Early-Phase Roles | Preclinical studies, toxicology, and BE/BA studies remain high due to rising drug-development pipelines and generics. | Charles River, Indian CROs (Accutest, Syngene, Lambda, Veeda) |
| Medical Writing & Documentation | Growing need for global submissions, regulatory documentation, study reports, and data reconciliation. | Parexel, ICON, Indian CROs with global/regional clients |
Whether you have coding and data-analysis skills, lab-based experience, or a background in life sciences/pharmacy/medical writing, there are ample avenues to explore within CROs in 2026.
What the Demand Looks Like and Why it’s Bigger than Ever
- The CRO services market continues to grow: outsourcing remains the preferred model for many sponsors because it’s cost-effective, scalable, and offers access to specialised expertise, especially for global trials across geographies.
- Asia-Pacific, notably India, is becoming a key hub for clinical trials: rising trial volume, regulatory maturity, and growing confidence in Indian CROs are driving more global sponsors to outsource here.
- Tech-enabled trials: With more decentralised trials, remote monitoring, real-world evidence (RWE) demands and hybrid/virtual trial models, CROs that can combine clinical operations with data-driven analytics and digital health infrastructure are growing fast.
- Niche & specialty-focused CROs are gaining prominence: As biotech and pharma push into rare diseases, oncology, biosimilars, generics, and global regulatory expansion, mid-size and specialised CROs (both global and regional) are seeing increased demand. This trend opens up diverse role types beyond traditional clinical research.
How to Find Job Openings for Clinical Research: Strategic Advice for 2026 Aspirants
If you’re considering a career in CROs, here’s a recommended roadmap:
- Monitor Company Career Pages & Job Portals: Global CROs like IQVIA, Parexel, ICON, Labcorp/Fortrea, Medpace often post fresh graduate/entry-level roles on their own career sites. Indian CROs (Syngene, Veeda, Lambda, Cliantha, Accutest, etc.) also post roles periodically.
- Leverage Professional Networks & LinkedIn: Many CROs encourage applicant referrals; connect with employees from CROs (India + global) and join pharma/clinical-research oriented professional groups.
- Gain Technical / Analytical Skills, Even Early: Skills such as SAS, statistical programming, medical writing, regulatory documentation, data management, or lab analytics boost employability considerably. Even entry-level or trainee roles often expect some comfort with data, clinical workflows or lab basics.
- Consider Regional / Local CROs for Start-ups: Smaller Indian CROs (or regionally based CROs) may have fewer applicants yet offer better learning exposure, more hands-on work, and faster growth, especially if you’re starting.
- Stay Updated with Industry Trends: Outsourced clinical research is evolving: decentralised trials, remote monitoring, hybrid designs, RWE-driven studies, specialty-disease trials. Position yourself in skill areas aligned with these trends (analytics, data, regulatory, remote-trial operations).
- Be Open to Bioanalytical + Lab-Based Roles: Especially if you have a background in life sciences, pharmacy, or lab sciences as these remain core to drug development pipelines.
At Academically, we provide industry-ready courses like a Post Graduate Certificate Courses to upskill in the dynamic environment. You can choose from Clinical Research, Pharmacovigilance, Clinical Data Management and so on. These help students build job-ready skills aligned with what CROs look for.
Final Thoughts
The world of clinical research is undergoing rapid transformation. As the pharmaceutical, biotech, and medical-device industries advance, CROs remain the backbone that enables them to conduct efficient, global-scale studies. This ranges from early discovery to post-market surveillance. For job-seekers and fresh graduates, 2026 presents one of the most promising windows in recent memory: higher demand, diversified roles, global exposure and growing acceptance of data and technology-oriented positions.
If you are passionate about drug development, global health, data-driven research, or clinical trials, consider exploring opportunities with the CROs listed above. With the right skills (data/biostatistics, regulatory understanding, lab or clinical background and the right readiness mindset, you could carve a meaningful career in this growing, impactful industry.
At Academically, you have the opportunity to shape that journey by equipping learners not just with knowledge but with job-ready skills, mentorship, and access to global CRO career paths. Let’s enable more graduates to become part of tomorrow’s breakthroughs, because behind every new therapy lies careful, rigorous clinical research, powered by CROs… and people like you.