Did you know that it takes nearly 10-12 years for a new drug to get to you from the time of its inception? And nearly 40% of this time is invested in the clinical research part of the drug development path.
So, what is clinical research? And why is it such an important part of new drug development?
In this blog, we will take you on a journey to see how a chemical entity goes from being a drug under development to a medicine you use.
Let’s begin!
What Exactly Is Clinical Research in Drug Development?
Before the stage of clinical research, researchers spend years in labs figuring out how a drug might work. But humans are not petri dishes. Here is where clinical research comes in to bridge the disconnect. In this stage, scientists test new drugs, therapies, or medical devices in people.
Clinical trials help answer:
- Does this drug work in real patients?
- What dose is safe?
- Are there side effects?
- Does it work better than existing treatments?
Without clinical research, diseases remain unsolved mysteries.
The Drug Development Journey
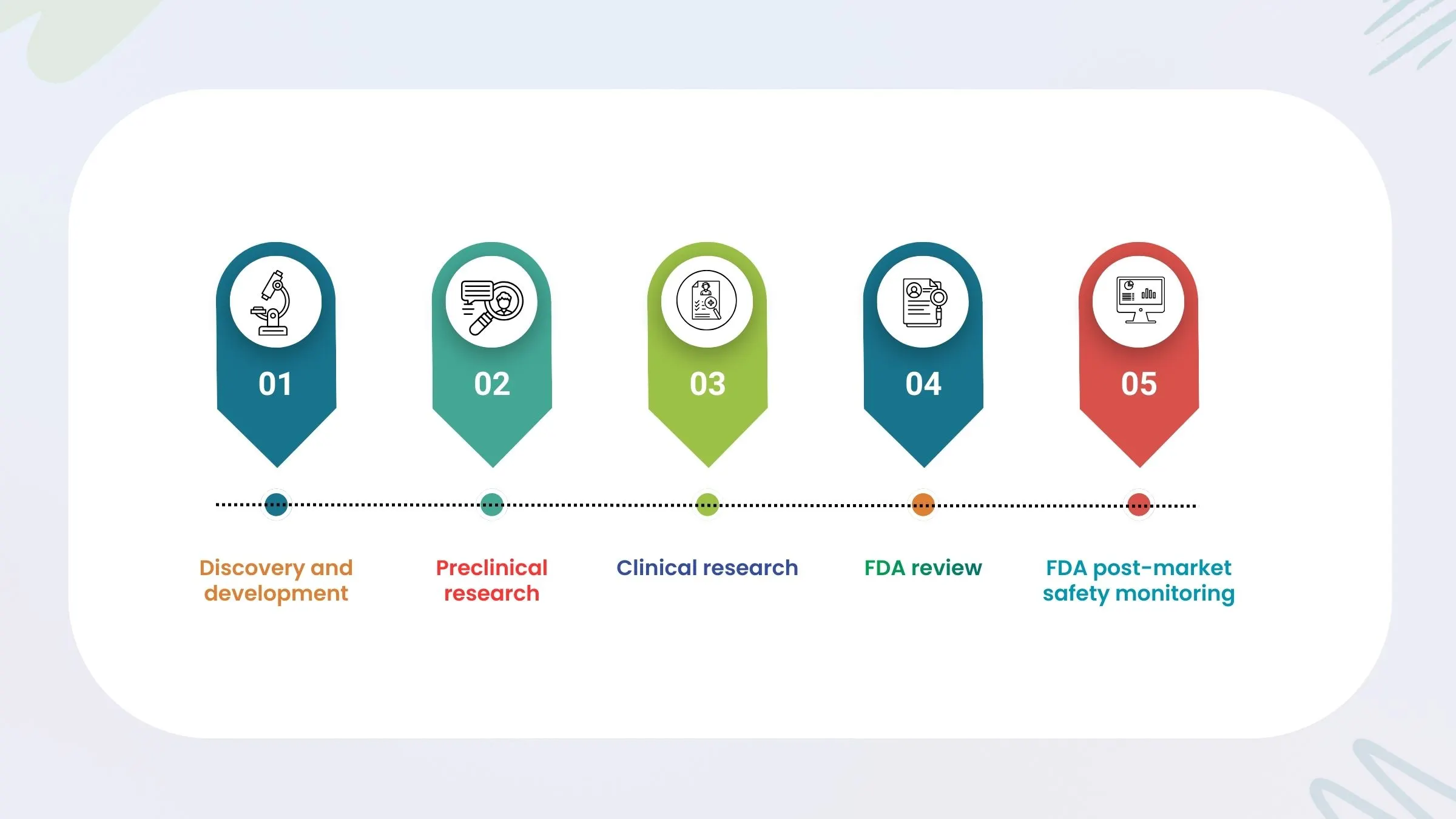
1. Discovery and Preclinical Phase
This is where the idea sparks. Scientists explore molecules and test them in labs and animals.
- Identify drug targets
- Study toxicity
- Understand how the drug behaves in the body
Only a few promising candidates survive this phase.
2. Clinical Trials - The Heart of Clinical Research
This is where volunteers and patients come in. Three main phases make or break the drug.
| Phase | Purpose | Participants | Key Question |
| Phase 1 | Test safety & dosage | 20–100 healthy volunteers | Is it safe? |
| Phase 2 | Test effectiveness | 100–300 patients | Does it work? |
| Phase 3 | Large-scale testing | 1,000–3,000 patients | How well does it work compared to standard care? |
If the data looks good, the drug moves to the next stage.
3. Regulatory Review
All data is sent to agencies such as the
- FDA- USA
- EMA- Europe
- CDSCO- India
They review:
- Safety
- Efficacy
- Manufacturing quality
Only after approval can a drug reach pharmacies.
4. Launch and Post-Marketing Surveillance (Phase 4)
Even after approval, clinical research continues.
Researchers track:
- Rare side effects
- Long-term benefits
- Real-world performance
This constant monitoring keeps patients safe.
Why Clinical Research Is the Backbone of Drug Development
Clinical research is what makes a drug reliable. It gives the data we can base our trust on. Without proof, a drug is just a hypothesis. Clinical research:
- Filters out unsafe treatments
- Identifies life-saving therapies
- Create better versions of existing drugs
- Builds confidence for doctors and patients
- Fuels innovation in biotech and pharma
It’s the scientific version of taking a car on a test drive, before letting millions buy it.
Real Examples of Clinical Research Changing the World
- COVID-19 vaccines were developed faster than ever because clinical research methods have become more efficient.
- Cancer immunotherapies evolved through breakthrough clinical trials that showed the immune system could be trained to fight tumors.
- Diabetes drugs like GLP-1 agonists like Ozempic, were refined through years of controlled clinical studies.
Behind every big medical win, there’s a team of
- Clinical researchers
- Coordinators
- Monitors
- Statisticians, and
- Thousands of volunteers
Job Opportunities in Clinical Research
There is no dearth of job opportunities in Clinical Research. You will find jobs across the industry, in:
- Pharma companies
- CROs
- Hospitals
- Biotech startups
Here’s a quick snapshot:
Popular Roles
- Clinical Research Coordinator
- Clinical Research Associate
- Medical Writer
- Data Manager
- Pharmacovigilance Associate
- Biostatistician
- Regulatory Affairs Specialist
- Clinical Project Manager
Salary Range (India and Global Snapshot)
| Role | India (INR per year) | US (USD per year) |
| CRC | ₹3–6 lakhs | $45,000–65,000 |
| CRA | ₹5–12 lakhs | $70,000–110,000 |
| Medical Writer | ₹4–10 lakhs | $60,000–95,000 |
| Safety/ PV Officer | ₹4–8 lakhs | $55,000–90,000 |
| Clinical Project Manager | ₹12–35 lakhs | $100,000–160,000 |
Why the hype? Because drug development isn’t slowing down. There is always something that is creating new demand:
- Chronic diseases
- Aging populations
- Biotech innovation
How Clinical Research Ensures Safety and Ethics
Watching over every trial:
- Ethics committees
- Regulatory authorities
- Data safety boards
They ensure:
- Volunteers are safe
- Patients give informed consent
- No shortcuts are taken
- Risks are minimized
- Data is accurate
This strict oversight is why modern medicines are some of the safest ever created.
Impact of Technology on Modern Drug Development
Clinical research has become smarter thanks to:
- AI-driven patient recruitment
- Wearables for real-time monitoring
- Electronic data capture
- Remote site monitoring
- Virtual trials
- Genomic testing for personalized treatments
This means faster trials and more targeted medicines.
Why You Should Care About Clinical Research
Clinical research is not just a phase. It is the cause of hope for many. The future of medicine depends on it. Imagine:
- Antibiotic-resistant infections
- Untreatable cancers
- Unknown viruses
- Autoimmune disorders with no cure
Clinical research is our best weapon. It gives hope where none existed.
A Career In Clinical Research
Clinical research is an essential part of the drug discovery process. It is what links the research done in the lab to real life efficacy of the product. The clinical research system is huge. There are people who are responsible for:
- Planning the trial
- Overseeing the trail
- Making reports
- Managing data
- Coordinating patients and medical staff
There is no dearth of job opportunities. And even if you don’t have experience or background in CR, it has become easier to enter the industry with certification courses in Clinical Research. These courses require less time commitment and are able to give the basic knowledge of the industry. You can become job ready in a much shorter amount of time.
So, when are you going to level up your career?





