Did you know that nearly 90% of healthcare decision-makers rely on Health Economics and Outcomes Research (HEOR) data before approving new treatments? That’s how crucial HEOR studies have become in shaping modern, evidence-based medicine. But what exactly are these studies, and how do they guide smarter, data-driven medical decisions? Let’s break it down in an easy-to-understand way.
What is HEOR and Why It Matters
Health Economics and Outcomes Research, or HEOR, might sound like a mouthful, but it’s actually pretty simple. Think of HEOR as the bridge connecting clinical outcomes, patient experiences, and healthcare costs. It doesn’t just ask, “Does this treatment work?” It also asks, “Is it worth it for patients, payers, and the healthcare system?”
In today’s world, where healthcare costs are rising and budgets are tight, HEOR is no longer optional. Pharmaceutical companies, insurance providers, and policymakers increasingly rely on HEOR data to make decisions that are both scientifically sound and economically sensible.
Why Evidence-Based Medicine Relies on HEOR
Evidence-Based Medicine (EBM) is all about making clinical decisions grounded in the best available evidence. Traditionally, this evidence comes from randomized controlled trials (RCTs), which focus on efficacy and safety. But RCTs often don’t answer a crucial question: Is the treatment worth the cost in the real world?
Here’s where HEOR steps in. By integrating economic evaluations, patient-reported outcomes, and real-world evidence, HEOR complements clinical research to create a fuller picture. It helps answer questions like:
- How much does a treatment cost per quality-adjusted life year (QALY) gained?
- Will adopting a new drug impact the overall healthcare budget?
- How do patients really feel and function after treatment?
In short, HEOR transforms raw clinical data into actionable insights for decision-makers.
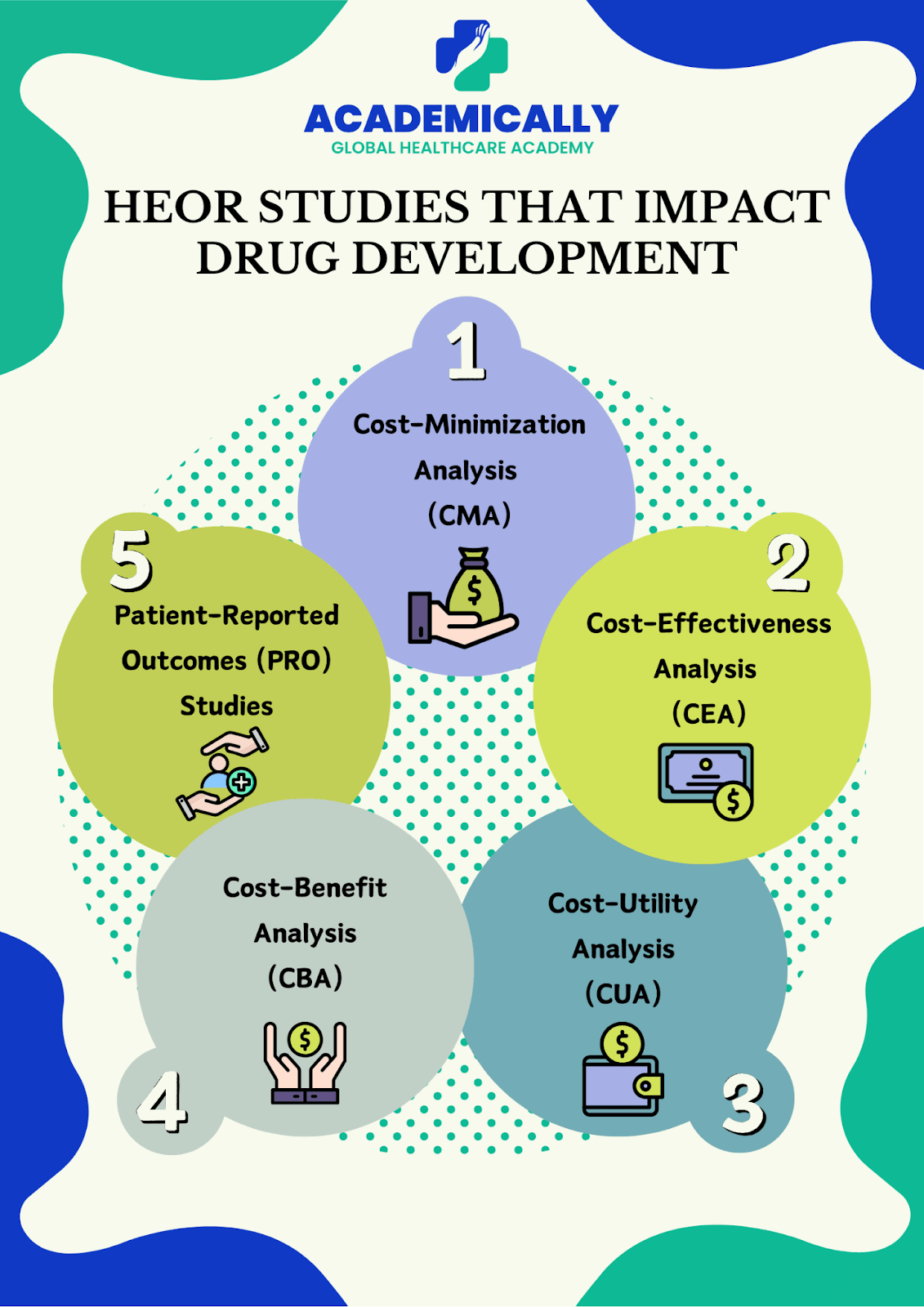
HEOR Studies that Impact Drug Development
HEOR isn’t one-size-fits-all. There are several types of studies, each with a unique purpose. Let’s explore the main ones:
1. Cost-Minimization Analysis (CMA)
CMA is the simplest economic study. It compares the costs of two or more interventions assuming their outcomes are identical. For example, if two drugs cure the same condition equally well, CMA tells you which is cheaper.
Use case: Choosing between generic and branded medications that have the same clinical effect.
2. Cost-Effectiveness Analysis (CEA)
CEA goes a step further. It evaluates both costs and health outcomes. Instead of just asking which treatment is cheaper, it asks: “How much health benefit do we get per dollar spent?”
Example: A study might compare two diabetes drugs based on cost per reduction in HbA1c levels.
Why it matters: CEAs help healthcare systems allocate resources efficiently, ensuring maximum health impact for every rupee spent.
3. Cost-Utility Analysis (CUA)
CUA is similar to CEA but uses quality-adjusted life years (QALYs) to measure outcomes. QALYs consider both quantity and quality of life, making this method particularly useful when treatments improve life quality rather than just survival.
Example: Evaluating a cancer therapy that extends life but has significant side effects. The therapy’s value isn’t just in added months but in how well patients live during those months.
Organizations like NICE (UK) heavily rely on CUA to decide which treatments to fund.
4. Cost-Benefit Analysis (CBA)
CBA goes even further by converting health outcomes into monetary terms. This allows direct comparison between costs and benefits in the same currency.
Example: Assessing whether a vaccination program saves more money in prevented hospitalizations than it costs to implement.
CBA is helpful for large-scale public health decisions, such as national vaccination strategies or screening programs.
5. Budget Impact Analysis (BIA)
BIA focuses on the financial impact of adopting a new therapy on a healthcare budget. Unlike CEA or CUA, which focus on cost per health outcome, BIA is about absolute budget implications over a defined period.
Example: A hospital wants to know how introducing a new biologic drug for rheumatoid arthritis will affect its pharmacy budget over the next five years.
BIA is especially important for insurance payers and healthcare administrators.
6. Patient-Reported Outcomes (PRO) Studies
PRO studies are all about the patient’s voice. They measure how patients feel, function, and experience their treatment. Unlike lab tests or imaging, PROs capture quality of life, symptom relief, and daily functioning.
Example: Evaluating how a migraine drug reduces days with headache and improves overall well-being.
PRO data are increasingly required for regulatory submissions, showing that patients’ perspectives matter in modern medicine.
7. Real-World Evidence (RWE) Studies
RWE studies analyze data from real-life settings, such as electronic health records, insurance claims, or patient registries. They complement traditional clinical trials by showing how treatments perform outside the controlled environment.
Example: Tracking the long-term effectiveness of a heart failure drug in diverse patient populations.
RWE is growing rapidly, particularly with digital health tools and AI analytics, which allow faster and more precise insights.
Real-World Applications of HEOR
HEOR isn’t just academic; it has real-world impact across the healthcare ecosystem:
- Pharmaceutical companies use HEOR to justify pricing and demonstrate a drug’s value to payers.
- Insurance companies rely on HEOR for formulary decisions and coverage policies.
- Governments and policymakers use HEOR to make public health decisions, such as vaccine programs or chronic disease management strategies.
Example: In oncology, HEOR data often determine whether a new expensive cancer therapy is reimbursed. Treatments with robust cost-effectiveness and patient outcome data are more likely to be approved.
HEOR and Policy: Shaping the Future of Healthcare
HEOR is central to value-based care, where reimbursement is tied to actual outcomes rather than just treatment volume. Many countries have established health technology assessment (HTA) bodies, like NICE (UK) and PBAC (Australia), which rely heavily on HEOR evidence.
HEOR also supports global healthcare sustainability. With rising costs, data-driven resource allocation ensures that limited funds achieve maximum patient benefit.
Challenges and the Road Ahead
Despite its importance, HEOR faces challenges:
- Data limitations: Real-world data can be messy and incomplete.
- Bias and variability: Different study designs can produce varying results.
- Integration with AI: While AI tools improve HEOR analysis, standardization and validation are needed.
Looking ahead, HEOR is poised to play a key role in precision medicine and patient-centered care, helping healthcare systems make smarter, fairer, and more sustainable decisions.
Conclusion
Health Economics and Outcomes Research is no longer a side tool it’s a cornerstone of evidence-based medicine. By combining clinical outcomes, economic evaluations, and patient perspectives, HEOR ensures that healthcare decisions are effective, sustainable, and patient-focused.
For anyone in pharma, policy, or clinical practice, understanding HEOR isn’t just helpful it’s essential. After all, in the world of modern healthcare, data-driven decisions save both lives and resources.