Overview of the Trial and Its Objectives
The trial named Purpose 1 involved 5,000 participants across three sites in Uganda and 25 sites in South Africa. This trial aimed to test the effectiveness of lenacapavir, a new drug given as an injection every six months, against two other daily PrEP pills used to prevent HIV. Lenacapavir works by breaking down a protein shell that HIV needs to reproduce. The trial was sponsored by Gilead Sciences and had several goals.
First, it aimed to see if a twice-yearly injection of LENACAPAVIR could protect women aged 16 to 25 better than the commonly used daily PrEP pill, Truvada. Second, it aimed to compare Descovy, a newer daily pill, to see if it was just as effective. The trial also studied the pharmacokinetics of the drugs, which means how the drug moves into, through, and out of the body.
Structure and Results of the Trial
The trial was conducted in a double-blind fashion, meaning neither the participants nor the researchers knew which treatment was being given until the end of the trial. Participants were randomly assigned to one of three groups in a 2:2:1 ratio to receive either the Lenacapavir injection, Descovy pill, or the Truvada pill.
During the randomised phase, none of the 2,134 women who received the Lenacapavir injection contracted HIV, showing 100% effectiveness. In comparison, 16 out of 1,068 women (1.5%) taking Truvada and 39 out of 2,136 women (1.8%) taking Descovy contracted the virus. Based on these results, an independent data safety monitoring board which was set up before the start of the trial, recommended ending the trial's blinded phase and giving all participants a choice of the treatment they wanted out of the 3.
Significance and Symptoms
Did you know that last year, approximately 1.3 million new HIV infections occurred worldwide, down from 2 million in 2010? This breakthrough offers new hope to patients by providing a highly effective way to prevent HIV.
PrEP is a key part of preventing HIV and should be combined with other strategies like HIV self-testing, access to condoms, screening and treatment for sexually transmitted infections, and contraception for women. Even with these ways to stop HIV, it's hard to keep people, especially young people, from getting it.
Getting an injection every six months might help more people follow their plan to stop HIV because it's easier than taking a pill every day.
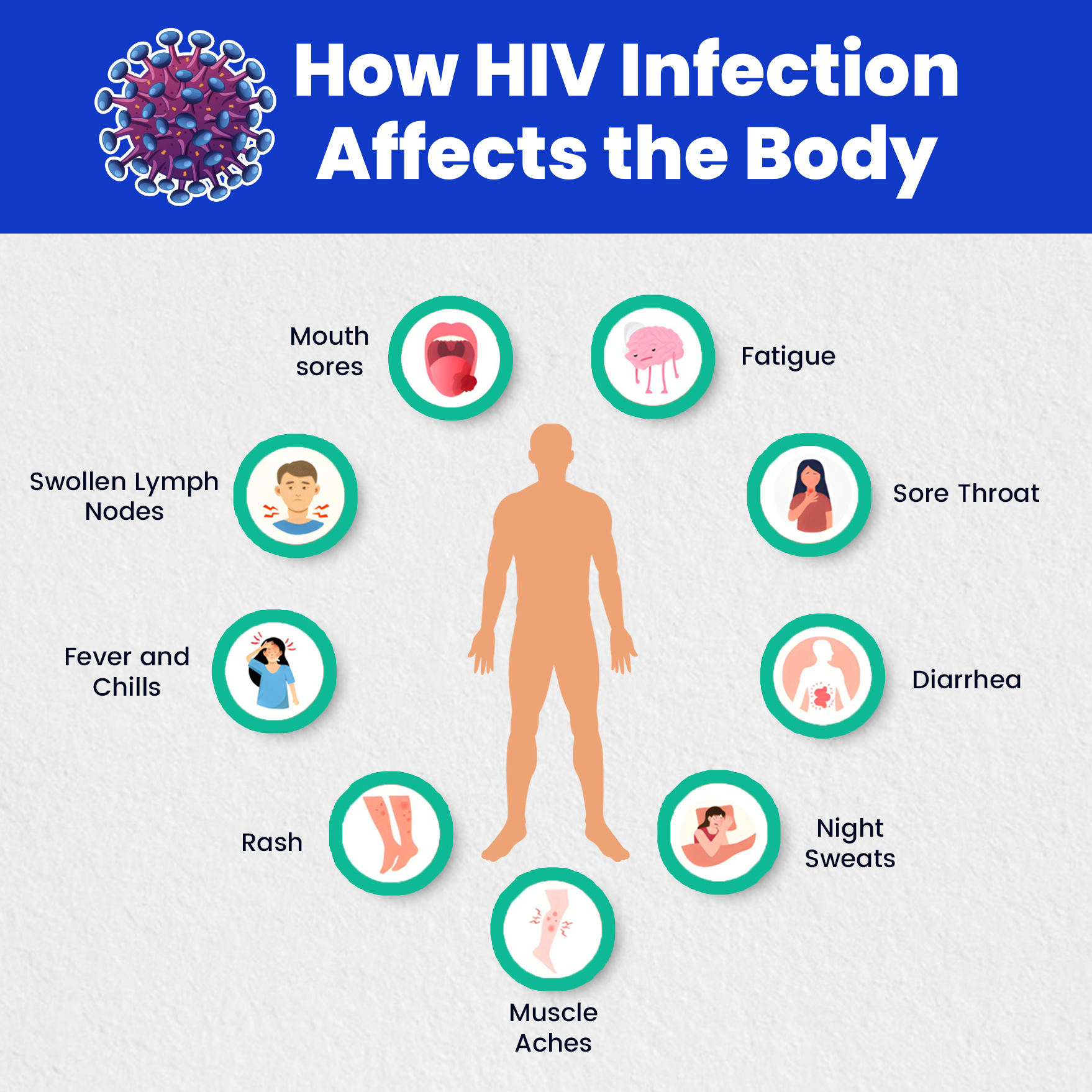
Acute HIV Infection (2-4 weeks after exposure)
- Fever
- Chills
- Rash
- Night sweats
- Muscle aches
- Sore throat
- Fatigue
- Swollen lymph nodes
- Mouth ulcers
Clinical Latency Stage (Chronic HIV)
- During this stage, the virus is still active but reproduces at very low levels.
- People may not have any symptoms or only mild ones. This stage can last for several years.
AIDS (Acquired Immunodeficiency Syndrome)
- Rapid weight loss
- Recurring fever or profuse night sweats
- Extreme and unexplained tiredness
- Prolonged swelling of the lymph glands in the armpits, groin, or neck
- Diarrhea that lasts for more than a week
- Sores of the mouth, anus, or genitals
- Pneumonia
- Red, brown, pink, or purplish blotches on or under the skin or inside the mouth, nose, or eyelids
- Memory loss, depression, and other neurologic disorders
It's important to note that having any of these symptoms doesn't necessarily mean someone has HIV. The only way to know for sure is to get tested.
Future Plans for HIV Prevention Trials
Going ahead, the Purpose 1 trial will enter an "open-label" phase. This means participants will know which treatment they're getting and can choose their preferred PrEP option. Meanwhile, another trial called Purpose 2 is ongoing. It's studying cisgender men, transgender people, and nonbinary individuals who have sex with men in different parts of Africa. Testing PrEP in diverse groups is crucial because its effectiveness can vary depending on factors like the type of sexual activity involved (anal, vaginal, or oral).
Regulatory Review
In the next few months, Gilead Sciences plans to show the trial results to government health officials in Uganda, South Africa, and other countries. They'll also share the data with the World Health Organization (WHO), which will review it and might make recommendations about how to use the drug.
If regulators approve the drug, it could become part of official guidelines from the WHO and national health authorities. After that, researchers will likely do more studies to figure out the best ways to use the drug in real-life situations.
Access and Affordability
Affordable access to this new PrEP option is essential for it to be widely used. Gilead Sciences intends to grant licences to generic drug manufacturers, which should help make the drug more affordable. This approach could enable governments to obtain the drug at lower costs and ensure it reaches everyone who needs protection against HIV.
Conclusion
In conclusion, the Purpose 1 trial's findings mark a significant step forward in HIV prevention, offering new hope and practical solutions for young women at high risk of HIV infection. With continued research, regulatory support, and efforts to ensure affordability, this breakthrough could play a groundbreaking role in the global fight against HIV/AIDS.
Let's work together to achieve UNAIDS' goal of fewer than 500,000 new infections by 2025 and potentially end AIDS by 2030.
For more in-depth information and to stay updated on the latest developments in HIV research and other medical advancements, visit Academically.